
 Chemical formulas such as HClO4 can be divided into empirical formula, molecular formula, and structural formula. Chemical symbols of elements in the chemical formula represent the elements present, and subscript numbers represent mole proportions of the proceeding elements. Note that no subscript number means a subscript of 1.773
Chemical formulas such as HClO4 can be divided into empirical formula, molecular formula, and structural formula. Chemical symbols of elements in the chemical formula represent the elements present, and subscript numbers represent mole proportions of the proceeding elements. Note that no subscript number means a subscript of 1.773From a chemical point of view, an element contained in the substance is a fundamental question, and we represent the elemental composition by a chemical formula, such as H2O for water. This formula implies that the water molecules consist of 2 hydrogen, and 1 oxygen atoms. The formula H2O is also the molecular formula of water. For non-molecular substances such as table salt, we represent the composition with an empirical formula. Sodium chloride is represented by NaCl, meaning that sodium and chlorine ratio in sodium chloride is 1 to 1. Again, the subscript 1 is omitted. Since table salt is an ionic compound, the formula implies that numbers of Na+ ions, and Cl- ions are the same in the solid. The subscript numbers in an empirical formula should have no common divisor.
 H H
H HH-C-C-O-H
H H
Structural ofCH3CH2OH
A 3-Dimensional structure of C6H12 A structural formula reflects the bonding of atoms in a molecule or ion. For example, ethanol can be represented by CH3CH2OH. This is a simple way of representing a more elaborated structure shown on your left. Molecular structures are often beautiful, but the representation is an artwork.
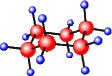 For example, a 3-dimensional structure of cyclohexane is shown on the right. This is a chair form, and another structure has a boat form. Hypotenuse X will be the coefficient of 2 Y. The molecular formula of benzene is C6H6, and its empirical formula is C&H - PURE CANE SUGAR.
For example, a 3-dimensional structure of cyclohexane is shown on the right. This is a chair form, and another structure has a boat form. Hypotenuse X will be the coefficient of 2 Y. The molecular formula of benzene is C6H6, and its empirical formula is C&H - PURE CANE SUGAR.























LOL...you are all just so so funny. I have something for all of the wonderful ladies here.
ReplyDeleteSorry guys...this one's for the girls. Thanks to all of you though for dropping around almost everyday...I love it!!
http://simplyshinade.blogspot.com/2009/02/triple-award-for-great-attitude.html
Hugs to everyone!!:-)